- 输入网页链接,自动生成快照
- 标签化管理网页链接
|
|
腹黑的火柴 · 青岛海信宽带多媒体技术有限公司(储备干部岗位 ...· 4 月前 · |
|
|
想旅行的钥匙 · Smoke DB log 5043429· 6 月前 · |
|
|
小眼睛的灭火器 · 成果推介-产线自动化、数字化、智能化升级—南 ...· 6 月前 · |
|
|
憨厚的大脸猫 · 小米笔记本Pro ...· 6 月前 · |
|
|
腹黑的槟榔 · 极端天气致印度比哈尔邦至少83人死亡-新华网· 7 月前 · |
|
|
含蓄的保温杯
7 月前 |

Global warming and globalization of trade have a decisive impact on the geographical distribution of insects. There is an exponential increase in the establishment of non-indigenous insects worldwide, along with the development of commercial networks. At the same time, global warming is promoting the expansion of many insect species northward and to higher altitudes. These two factors combined explain the multiple insect invasions observed in recent years.
- 1. An explosion of the establishment of non-native insects in Europe
- 2. Non-native species are spreading faster and faster across Europe
- 3. Global warming is changing the range of insects
- 4. Annual increase in average temperature, a relevant variable?
- 5. Expansion to the North and contraction to the South?
- 6. Globalization and global warming act in a combined way
- 7. Global warming alone is responsible for the expansion of insects?
- 8. Messages to remember
1. An explosion of the establishment of non-native insects in Europe
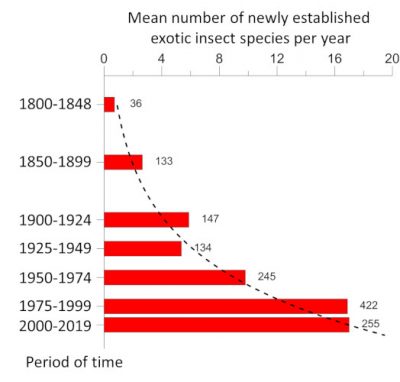
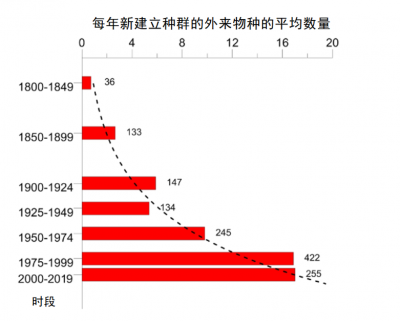
While the destruction of natural habitats is the primary cause of the current decline in biodiversity, invasions of non-native species are in second place. The rate of establishment of non-native insect species, i.e. introduced on a continent other than that of origin, has increased exponentially since the beginning of the 20 th century. Thus, in Europe, the number of newly established species increased from 9.7 per year during the period 1950-1974 to 17.0 during the period 1990-2016 [1] (Figure 1). By the end of 2016, 1418 non-native insect species had been identified as introduced and established in Europe [2] , making it the 2 nd largest group of non-native species after plants [3] (see When invasive plants also wander in the fields ). Similar values are observed on other continents [4] .

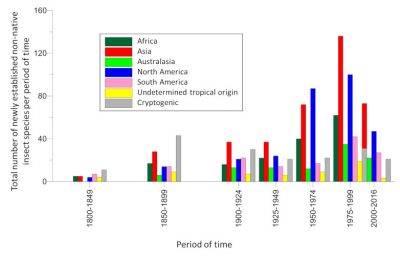
Nowadays, species qualified as “ emergent ” (because they have never previously been introduced in a continent other than the one of origin [6] ) have become predominant. This is the case, for example, of the Asian hornet detected in France in 2004.
2. Non-native species are spreading faster and faster across Europe
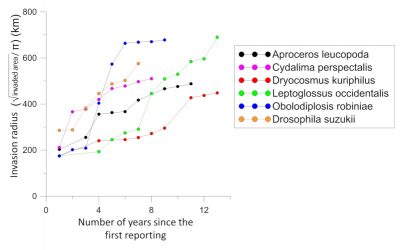
There is an acceleration in the rate of expansion of non-native insects . Non-native species that have become established in Europe over the past two decades have a faster average rate of expansion than those that arrived just after World War II [1] . Many of them colonized almost the entire continent in less than fifteen years, while most species established in the first half of the 20 th century took several decades to achieve this. Several examples indicate that this expansion rate is independent of the origin of the introduced insect (Figures 4 and 5).

Insects of Asian origin have spread even more rapidly, colonizing most of Europe in less than 10 years:
This recent acceleration in the expansion of non-native insects (Figure 5) could be linked to the political and economic changes in Europe (fall of the Berlin Wall and enlargement of the European Union), with in particular the abolition of intra-Community customs controls [1] .

3. Global warming is changing the range of insects
With an increase in global average temperature of 0.61°C since the beginning of the 20 th century and a future warming estimated at between 2.6 and 4.8°C for 2100 according to the models of the Intergovernmental Panel on Climate Change (IPCC [12] ), we can expect a significant impact of climate change on most insect species . This foreseeable impact is linked to the very biology of insects.
Insects are “cold” blooded animals that do not produce heat. They are called “ectotherms” unlike “endothermic” mammals, which are able to produce heat through their metabolic activity and keep their body temperature more or less constant. The body temperature of insects and the associated physiological processes will therefore depend directly on changes in ambient temperature. These variations will, among other things, govern their periods and rhythms of activity, the duration of their different stages of larval development and the number of annual generations, their flight possibilities, etc…. For a large number of species, specific temperature thresholds delimit barriers limiting their geographical distribution:

However, a simple comparison of the past and current distribution of species with the movement of isotherms during the same period is not sufficient to prove the implication of climate change. To demonstrate that this cannot result from a simple correlation, it is essential to identify a causal link between a change in the range and a change in the climatic factor considered.

4. Annual increase in average temperature, a relevant variable?
Global warming is most often assessed from the increase in the average annual air temperature at sea level. However, insect populations are not confronted with an average temperature, but with a daily variation in the weather conditions under which they develop. Taking into account only the increase in annual temperature may mask the differential effects of seasonal warming.
Thus, an increase in temperature in winter, spring, summer, or fall will not have the same biological impact as a result of allowing populations to expand or not . While the increase in winter temperatures allows some species to survive in previously unfavourable areas, an increase in spring and/or summer temperatures will tend to accelerate the development of immature stages, allowing an increase in the number of annual generations in some species, and encourage movement.

5. Expansion to the North and contraction to the South?
Few data exist on the possible contraction of the distribution area to its southern limit with increasing temperatures. In most European butterflies, the southern limit of northward moving species appears to have remained stable , and only a few, such as the lycaenid butterfly Heodes tytirus , show a decline in populations south of the area [15] . For two species of moths ( Lymantria dispar and L. monacha ), simulations based on the foreseeable increase in temperatures in the future suggest a retraction of the southern limit from 100 to 900 km due to the negative effects of excessively high temperatures on larval hatching from eggs in winter diapause [22] , a phenomenon that corresponds to a temporary pause in development in response to the occurrence of adverse climatic conditions.
More generally, for insects developing during spring and summer, changes in range could be roughly determined by a combination of development rate (fast or slow) and winter diapause process [23] :
6. Globalization and global warming act in a combined way

7. Global warming alone is responsible for the expansion of insects?
Most studies to date have focused on the effects of rising temperatures. But climate change incorporates multiple other factors, such as humidity, rainfall intensity and frequency, radiation, or greenhouse gas levels, which can act directly or in synergy with rising temperatures. For example, the establishment in our regions of the asian tiger mosquito, Aedes albopictus , is conditioned by temperature but also by the length of the day compared to the night (photoperiod), humidity and rainfall [28] . But the other studies, most often conducted under laboratory conditions, so far give only often contradictory indications as to the possible responses of insects to fluctuations in these variables, for example on the effect of CO 2 [29] . The development of large-scale field experiments appears essential.
Research on the relationship between climate change and insect expansion has often focused on the specific responses of insects with high economic or health impacts, rarely taking into account their associated organisms. However, climate change simultaneously affects the entire ecosystem, including host (plants), competing insects and natural enemies . The responses of each of these components may differ for the same temperature increase and the interactions may be significantly altered in one direction or another. For example, studies suggest that the effectiveness of some predators, such as ladybeetles, may increase in a warmer environment, and the expansion of aphid-prey may be reduced [30] . On the other hand, the pine processionary progresses faster northwards than its natural enemies [31] . The development of integrated studies, considering all ecosystem components simultaneously, is a necessity for future research.
8. Messages to remember
Notes and references
Cover image. Massive attack by the pine processionary at its altitudinal expansion front in the Italian Alps. Oulx, Piedmont; winter 2015-2016. [Source: Photo Roques © INRA]
[1] Roques A., Auger-Rozenberg M.-A., Blackburn T.M., Garnas J.R., Pyšek P. et al. (2016) Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biological Invasions 18 , 907-920.
[2] Source: DAISIE European project database (“ Delivering Alien Invasive Species Inventories in Europe”; http://www.europe-aliens.org) recently updated in the EASIN database (“ European Alien Species Information Network “; http://easin.jrc.ec.europa),
[3] DAISIE (2009) Handbook of alien species in Europe . Berlin: Springer Science + Business Media B.V.
[4] Seebens H., Blackburn T.M., Dyer E.E., Genovesi P., Hulme P.E. et al. (2017) No saturation in the accumulation of alien species worldwide. Nature Communications . DOI: 10.1038/ncomms14435.
[5] Roques A., Rasplus J.Y., Rabistch W., Lopez-Vaamonde C., Kenis M et al. (2010). Alien Terrestrial Arthropods of Europe. BioRisk 4 (1 and 2), 1-1024.
[6] Seebens A., Blackburn T.M., Dyer E.E., Genovesi P., Hulme P.E. et al. (2018). The global rise in emerging alien species results from increased accessibility of new source pools. Proceedings of the National Academy of Sciences of the USA. doi/10.1073/pnas.1719429115.
[7] Lesieur V., Lombaert E., Guillemaud T., Courtial B., Strong W. et al. (2018) The rapid spread of Leptoglossus occidentalis in Europe: a bridgehead Invasion. Journal of Pest Science. https://doi.org/10.1007/s10340-018-0993-x
[8] B ras A. Invasion fulgurante de la pyrale du buis en Europe: Caractérisation de la diversité génétique de Cydalima perspectalis (Walker, 1859) et approche phylogéographique https://belinra.inra.fr/doc_num.php?explnum_id=1021 (in french)
[9] Cini A., Ioriatti C., Anfora G. (2012). A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bulletin of Insectology 65 (1) 149-160.
[10] Desneux N., Luna M.G., Guillemaud T. and Urbaneja A. (2011) The invasive South American tomato pinworm, Tuta absoluta , continues to spread in Afro-Eurasia and beyond the new threat to tomato world production. Journal of Pest Science 84 , 403 – 408.
[11] Javal M., Roques A., Haran J., Herard F., Keena M., Roux G. (2017) Complex invasion history of the Asian long-horned beetle: fifteen years after first detection in Europe. Journal of Pest Science DOI 10.1007/s10340-017-0917-1.
[12] http://ar5-syr.ipcc.ch/
[13] Ungererer M.J., Ayres M.P., Lombardero M.J. (1999). Climate and the northern distribution limits of Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). Journal of Biogeography 26 , 1133-45.
[14] Faucet C., Rousselet J., Pineau P.; Miard F., Roques A. (2013). Are heat waves susceptible to mitigate the expansion of a species progressing with global warming? Ecology and Evolution 3 (9), 2947-57.
[15] Parmesan C, Ryrholm N, Stefanescu C et al (1999). Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399 , 579-83.
[16] Parmesan C (1996). Climate change and species’ range. Nature 382 , 765-766.
[17] Hickling R., Roy D.B., Hill J.K., Fox R., Thomas C.D. (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology 12 , 450- 455.
[18] Trân J.K., Ylioja T., Billings R.F., Régnière J., Ayres M.P. (2007). Impact of minimum winter temperatures on the population dynamics of Dendroctonus frontalis . Ecological Applications 17 , 882-899.
[19] Jepsen J.U., Hagen S.B., Ims R.A., Yoccoz N.G. (2008). Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. Journal of Animal Ecology 77 , 257-264.
[20] Chapman J.W., Reynolds D.R., Smith A.D., Riley J.R., Pedgley D.E., Woiwod I.P. (2008). Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Current Biology 18 , 514-518.
[21] Battisti A. Stastny M., Buffo E., Larsson S. (2006). A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Global Change Biology 12 , 66- 671.
[22] Vanhanen H., Veteli T.O., Pailvinen S., Kellomaki S., Niemala P. (2007). Climate change and range shifts in two insect defoliators: Gypsy moth and nun moth – A model study. Silva Fennica 41 , 621-38 ;
[23] Bale J.S., Masters G.J., Hodkinson I.D. et al. (2002). Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology 8 , 1-16.
[24] Eschen R., Roques A., Santini, A. (2015). Taxonomic dissimilarity in patterns of interception and establishment of alien arthropods, nematodes and pathogens affecting woody plants in Europe. Diversity and Distributions 21 (1), 36-45.
[25] Walther G R., Roques A., Hulme P.E., Sykes M.T., Pyšek P., et al. (2009). Alien species in a warmer world: risks and opportunities. Trends in Ecology and Evolution 24 , 686-692.
[26] Roques A. (2010). Alien forest insects in a warmer world and a globalized economy: Impacts of changes in trade, tourism and climate on forest biosecurity. New Zealand Journal of Forestry 40 , 77-94.
[27] Ott J., ed (2008). Monitoring climate change with dragonflies. Series Faunistica 81. Pensoft, Sofia. Cannon R.J.C. (1998). The implications of predicted climate change in the UK, with emphasis on non-indigenous species. Global Change Biology 4 , 785- 796.
[28] Eritja R., Escosa R., Lucientes J. et al. (2005). Worldwide invasion of vector mosquitoes: Present European distribution and challenges for Spain. Biological Invasions 7 , 87-97.
[29] Newman J.A. (2005). Climate change and the fate of cereal aphids in Southern Britain. Global Change Biology 11 , 940-944.
[30] R.J.C. Cannon (1998). The implications of predicted climate change in the UK, with emphasis on non-indigenous species. Global Change Biology 4 , 785-796.
[31] Auger-Rozenberg M.A., Barbaro L., Battisti A., Blache S.,. Charbonnier Y., et al. (2015). Ecological responses of parasitoids, predators and associated insect communities to the climate-driven expansion of pine processionary moth. pp. 311- 358 In Roques A. (Ed.) Processionary Moths and Climate Change: An Update. Springer/ Quae
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie ( www.a3e.fr ), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: ROQUES Alain, AUGER-ROZENBERG Marie-Anne (August 16, 2019), Climate change and globalization, drivers of insect invasions, Encyclopedia of the Environment, Accessed August 24, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/climate-change-globalization-drivers-of-insect-invasions/ .
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.
Author(s)
Article outline
- 1. An explosion of the establishment of non-native insects in Europe
- 2. Non-native species are spreading faster and faster across Europe
- 3. Global warming is changing the range of insects
- 4. Annual increase in average temperature, a relevant variable?
- 5. Expansion to the North and contraction to the South?
- 6. Globalization and global warming act in a combined way
- 7. Global warming alone is responsible for the expansion of insects?
- 8. Messages to remember
Focus
Learn more
When invasive plants also wander in the fields
21-03-2019
Biological invasions are easily perceived by the general public, who see the development of populations…
Bruno CHAUVEL , Research Officer at INRA, Unité d’agroécologie de l’INRA, INRA Dijon
The adaptation of life to environmental constraints
08-04-2019
The diversity of living forms, or biodiversity, is reflected in phenotypic variations (expression of variable…
Laurence DESPRÉS , Professor, Laboratoire d'Ecologie alpine, Université Grenoble Alpes
Biodiversity is not a luxury but a necessity
02-03-2020
Biodiversity, a contraction of the term “biological diversity”, appeared in the public sphere at the…
Jacques BLONDEL
, Emeritus Research Director at the CNRS, CEFE, Montpellier
PDF

全球变暖和贸易全球化对昆虫的地理分布具有决定性的影响。随着商业网络的发展,世界各地非本土昆虫种群建立的情况呈指数增长。同时,全球变暖正促使许多昆虫向北扩散到更高纬度的地区。这两个因素共同解释了近年出现的多次昆虫入侵现象。
1. 欧洲非本地昆虫种群建立的爆炸式增长
当满足以下条件时,可认定一个物种构成 入侵 :(a)从外界(其发源地大陆以外)引进,(b)在本地建立种群(成功繁殖),然后(c)扩散至登陆地之外,并造成(d)一系列负面影响(经济、健康、社会),破坏当地生态系统的稳定。
尽管自然栖息地的破坏是目前生物多样性下降的主要原因,但非本地物种入侵已经成为第二大威胁。自20世纪初以来,非本地昆虫物种(即被引进至其发源地以外的大陆的昆虫)建立种群的速度呈指数增长。在欧洲新建立种群的物种数量从1950-1974年期间的每年9.7个,增至1990-2016年期间的17.0个 [1] (图1)。到2016年底,确定引进并于欧洲建立种群的非本地昆虫物种达1418种 [2] ,使其成为仅次于植物的第二大类非本地物种 [3] (参阅“当入侵植物也在田间蔓延”)。在其他大陆上也出现了类似的情况 [4] 。

这种传播速度的加快在很大程度上(> 90%)是由 与人类活动有关的意外介入引起 ,并且主要与全球 观赏植物贸易 的快速增长有关,因为这让植物相关物种得以在全球运输 [5] 。然而,也有物种的传播与特定的生物学原因无关,例如通过容器或货物被引入,例如亚洲虎蚊(白纹伊蚊)或亚洲墨胸胡蜂(图2)。
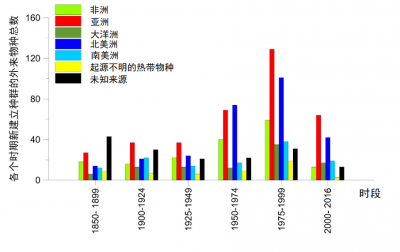
当前,非本地昆虫物种的自发入侵数量一直保持稳定 [4] 。但由于新市场、新销路的拓展,以及运输货物本身的特性,昆虫的输入来源显著扩大。因此, 亚洲 最近成为 入侵 欧洲昆虫 物种的主要来源地 (>30%),取代了此前的主要来源地北美(图3)。
如今,(某些)定性为“ 新兴 ”的物种(称为“新兴”的原因是它们此前从未被引入至起源地以外的大陆 [6] )已成为入侵地的优势物种。例如2004年在法国发现的亚洲大黄蜂就是这种情况。
2. 非本地物种在欧洲的传播速度越来越快
非本地昆虫扩张速度 正在 加快 。过去二十年,在欧洲建立种群的非本地物种的平均扩张速度比起二战后刚进入时明显加快 [1] 。在不到十五年的时间里,许多入侵物种已几乎遍布整个欧洲大陆,然而,这在20世纪上半叶要花费数十年才能完成。部分案例表明,这种扩张速度与所入侵昆虫的来源地并不相关(图4和5)。

来自北美原生地的西部喙缘蝽( Leptoglossus occidentalis )(图4),于1999年在意大利东北部首次发现 [7] 。在2018年,它便扩散到葡萄牙、俄罗斯、从北非到斯堪的纳维亚半岛,以及中东、亚洲和南美洲。
亚洲起源的昆虫传播速度甚至更快,以不到10年的时间席卷了欧洲大部分地区,这些物种有:
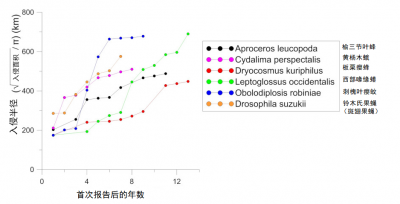
对过去250年中来到欧洲的1171种非本地昆虫进行分析表明,1990年后引入物种的初始扩张速率:
近期非本地昆虫扩张速度的加快(图5)可能与 欧洲的政治和经济变化 (柏林墙倒塌和欧盟的扩大)有关,尤其是欧盟内部的海关管制的取消 [1] 。
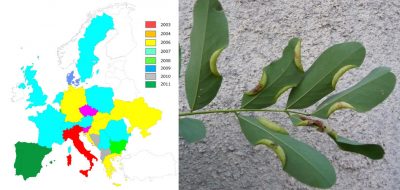
许多昆虫可自然传播,有时传播的距离很远,但由于入侵区域是逐年扩展的,对大多数被研究的物种而言,将自然传播作为合理解释仍比较难令人信服。例如:

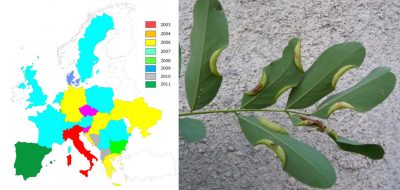
对于部分物种而言,入侵地区和原生地区之间的种群遗传多样性比较表明:存在同一种昆虫不同原生地区的多源引入的情况。也可能涉及“桥头堡(Bridgehead)”现象,即引入的昆虫来自于已入侵的地区 [7] 。这种多源引入可解释某些物种的快速传播,例如黄杨木蛾 [8] 、西部喙缘蝽 [7] 和亚洲长角甲虫(光肩星天牛, Anoplophora glabripennis ) [11] (图7)。然而,遗传分析也表明: 人类活动,特别是植物贸易 ,对这些非本地昆虫在欧洲的快速传播 起着关键的作用 。黄杨木蛾便是一个典型案例:其卵和毛虫通过苗圃和花卉中心的观赏黄杨木贸易传播至整个欧洲 [8] (图4)。
3. 全球变暖正在改变昆虫的分布范围
根据政府间气候变化专门委员会(IPCC [12] )的模型,自20世纪初以来全球平均温度升高了0.61°C,2100年时变暖导致的升温将达到2.6至4.8°C之间,由此可以预见气候变化将 对大多数昆虫物种产生显著影响 。这种可预见的影响与昆虫的生物学特性直接相关。
昆虫是不产生热量的“冷血”动物。与“内温型(endothermic)”哺乳动物通过新陈代谢产生热量并保持体温恒定不同,昆虫被称为“外温动物”,其体温和有关生理过程直接取决于环境温度的变化。这些变化与其他因素一起,共同决定它们的活动周期和节奏、幼虫发育的不同阶段所持续的时间和年世代数,以及它们的飞行可能性等。对于许多物种, 特定的温度阈值 会解除其地理分布的限制:

但是,将物种过去和当前的分布与同时期等温线的移动进行简单比较,并不足以证明气候变化的影响。为了证明这不是简单相关性造成的,我们必须确定分布范围变化与所考虑的气候因素变化之间的因果关系。

在温带地区, 低温通常是限制昆虫物种分布范围的关键因素 。昆虫冬季的生存以及发育阶段的转化(例如卵、幼虫、若虫和成虫)都存在最低温度阈值。 因此,冬季温度略微的升高即会使其能在原先因恶劣的气候条件无法生存的地区存活 :
4. 年平均气温的升高,是一个与之有关的变量吗?
全球变暖最常用的评估方法是根据海平面平均气温的升高进行评估。然而,昆虫种群并不是依据平均温度,而是依据其生活环境所处天气条件下的每日变化(温度)来进行评估。仅考虑年度气温升高不够全面,不足以探明季节性变暖(造成)的不同影响。
因此, 春、夏、秋、冬四季的升温在种群扩大与否上所造成的生物学影响并不相同 。冬季温度的升高使某些物种能够在先前不利的地区生存,春季和/或 夏季温度的升高将可能加速其未成年阶段的发展,从而使某些物种的年世代数增加,进而为其迁移创造出有利条件。
对于迁徙性的蝴蝶物种尤其如此,例如夜蛾 Autographa gamma ,温度升高帮助它们从北非的越冬点入侵至英国 [20] 。
在意大利阿尔卑斯山,2003年夏季的热浪也促使松异舟蛾扩展其分布范围。因为夜晚温度超过了雌蛾飞行所需要阈值(14°C)的天数达到了正常值的5倍,这也导致了更多的飞蛾散布到更远的地区 [21] 。

然而,年温度升高也可能掩盖了 季节性变暖所造成的相反作用(contradictory effects) ,例如日本的稻绿蝽( Nezara viridula )(图10)。这种虫子能否向北扩张,取决于全球变暖正面和负面效应是否达到平衡:冬天成虫存活率提高,秋季加速未成年幼虫发育是正向效应,但夏季对幼虫发育会产生负面影响。同样,尽管2002-2003年显著的冬季变暖使松异舟蛾幼虫能够在巴黎地区扩张和生存,但2003年夏季的热浪使这个扩张的种群消失了很大一部分 [13] 。因此,对于未来频率还会有所上升的极端事件(例如夏季热浪),其额外影响也应予以考虑,尽管目前我们对此知之甚少 [17] 。
5. 北扩南缩?
很少有数据表明随着温度的升高,昆虫的南端分布界限区域会收缩。大多数的欧洲蝴蝶中, 向北移动的物种的南端分布界限似乎仍保持稳定 ,只有极少数,例如灰蝶( Heodes tytirus ),在南部地区的数量减少 [15] 。基于未来可预见的升温模拟表明,对于两种蛾(舞毒蛾 Lymantria dispar 和模毒蛾 L . monacha ),由于 滞育( diapause ) (一种因应不利的气候条件而暂停发育的现象),这一过高温度将对卵孵化幼虫产生负面影响 [22] ,使其 南端分布界限缩回 100到900 km的距离。
一般而言,对于在春季和夏季发育的昆虫,其分布范围的变化大致由发育速度(快或慢)和冬季滞育状态共同决定 [23] :
6. 全球化与全球变暖协同作用
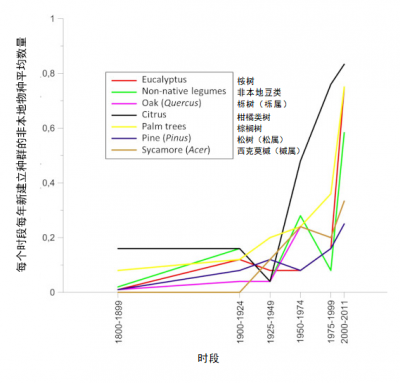
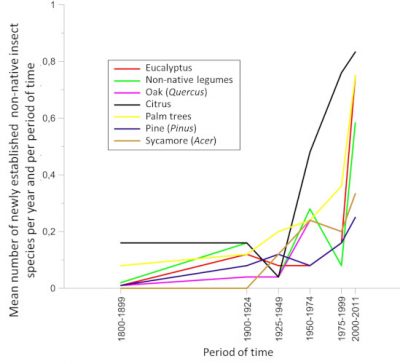
贸易的全球化促使亚热带或热带地区的昆虫物种意外来到欧洲大陆。然而,直到最近它们的种群建立仍受到限制:冬季天气不利于其在大陆大部分地区生存,利于其发育的温度条件持续时间有限。但是 ,自20世纪90年代以来,在欧洲种植的热带或亚热带起源的植物 (如桉树,棕榈树和其他合欢树(acacias))上, 非本地昆虫的种群建立相较过去有明显增长 [24] (图11)。到本世纪00年代末,来自亚热带或热带气候区在欧洲建立种群的昆虫已有400多种 [25] 。这些物种分布通常仅限在引进地,常在地中海地区,但是近年来,与棕榈树有关的昆虫入侵现象时有发生(图12),也进一步说明这些物种的分布情况也有所改变。

虽然直到本世纪00年代中期,红棕象甲( Rhynchophorus ferrugineus )和棕榈蝶蛾( Paysandisia archon butterfly )一直局限分布于在西班牙南部和加那利群岛的传入地带。但在2004年至2007年期间(图12),它们就占领了地中海大部分地区,棕榈蝶蛾的分布区域甚至还远至英格兰南部。之所以会出现这种迅速的蔓延,是因为全球变暖产生了综合影响,让这些热带物种得以更好地发育,而观赏性棕榈的贸易发展也是一大原因。随着用于装饰雅典奥运会(2004年)的西班牙棕榈树的进口,红棕象甲也随之到来 [26] 。类似情况还有 本地昆虫 由人类意外地 运输 至超出其自然分布范围以外的地区。这些引入地区新的气候条件使它们得以在本地扎根,特别是那些被向北运输的地中海昆虫,例如欧洲螳螂(薄翅螳螂, Mantis religiosa ) [27] 或松异舟蛾( Thaumetopoea pityocampa ,参阅“松异舟蛾:全球变暖影响的典型”)。
7. 仅仅全球变暖是造成昆虫扩散的原因吗?
迄今为止,大多数研究都集中在升温的影响上。但是,气候变化还包含许多其他因素,例如湿度、降雨强度和频率、辐射或温室气体水平,这些因素可以直接发挥作用,也可以与升温产生协同作用。例如,在我们(法国)地区,亚洲虎蚊(白纹伊蚊, Aedes albopictus )的种群建立受到温度的影响,但同样还受到昼夜长短(光周期)、湿度和降雨的限制 [28] 。但是其他一些在实验室条件下进行的研究,像昆虫对二氧化碳等各种变量波动的可能响应等,通常会得出相互矛盾的结果 [29] 。因此,开展大规模野外实验显得尤为必要。
关于气候变化和昆虫扩散之间关系的研究,通常集中在对经济或健康有重大影响的昆虫的具体响应上,很少考虑与其相关的其他生物。然而, 气候变化会同时影响整个生态系统,包括宿主(植物)、竞争性昆虫和天敌 。对于相同程度的升温,(生态系统的)不同组分的响应可能会有所不同,并且相互的作用可能会在一个方向或另一个方向上发生显著变化。例如研究表明:某些捕食者 (predators)的效力(effectiveness)可能会增强,例如瓢虫在温暖的环境中可能会增加(效力),蚜虫(猎物)的扩张可能会减慢 [30] 。另一方面,松异舟蛾比其自然天敌向北扩张得更快 [31] 。未来,开展同时考虑生态系统所有组分的整合研究非常必要。
8. 重要的信息(Messages to remember)
参考资料及说明
封面图片: 2015-2016年冬季在意大利皮埃蒙特,阿尔卑斯山乌尔克斯(Italian Alps. Oulx, Piedmont)松异舟蛾沿纵向扩张的大规模攻击。 [来源:Photo Roques © INRA]
[1] Roques A., Auger-Rozenberg M.-A., Blackburn T.M., Garnas J.R., Pyšek P. et al. (2016) Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biological Invasions 18 , 907-920.
[2] Source: DAISIE European project database (“ Delivering Alien Invasive Species Inventories in Europe”; http://www.europe-aliens.org) recently updated in the EASIN database (“ European Alien Species Information Network “; http://easin.jrc.ec.europa),
[3] DAISIE (2009) Handbook of alien species in Europe . Berlin: Springer Science + Business Media B.V.
[4] Seebens H., Blackburn T.M., Dyer E.E., Genovesi P., Hulme P.E. et al. (2017) No saturation in the accumulation of alien species worldwide. Nature Communications . DOI: 10.1038/ncomms14435.
[5] Roques A., Rasplus J.Y., Rabistch W., Lopez-Vaamonde C., Kenis M et al. (2010). Alien Terrestrial Arthropods of Europe. BioRisk 4 (1 and 2), 1-1024.
[6] Seebens A., Blackburn T.M., Dyer E.E., Genovesi P., Hulme P.E. et al. (2018). The global rise in emerging alien species results from increased accessibility of new source pools. Proceedings of the National Academy of Sciences of the USA. doi/10.1073/pnas.1719429115.
[7] Lesieur V., Lombaert E., Guillemaud T., Courtial B., Strong W. et al. (2018) The rapid spread of Leptoglossus occidentalis in Europe: a bridgehead Invasion. Journal of Pest Science. https://doi.org/10.1007/s10340-018-0993-x
[8] B ras A. Invasion fulgurante de la pyrale du buis en Europe: Caractérisation de la diversité génétique de Cydalima perspectalis (Walker, 1859) et approche phylogéographique https://belinra.inra.fr/doc_num.php?explnum_id=1021 (in french)
[9] Cini A., Ioriatti C., Anfora G. (2012). A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bulletin of Insectology 65 (1) 149-160.
[10] Desneux N., Luna M.G., Guillemaud T. and Urbaneja A. (2011) The invasive South American tomato pinworm, Tuta absoluta , continues to spread in Afro-Eurasia and beyond the new threat to tomato world production. Journal of Pest Science 84 , 403 – 408.
[11] Javal M., Roques A., Haran J., Herard F., Keena M., Roux G. (2017) Complex invasion history of the Asian long-horned beetle: fifteen years after first detection in Europe. Journal of Pest Science DOI 10.1007/s10340-017-0917-1.
[12] http://ar5-syr.ipcc.ch/
[13] Ungererer M.J., Ayres M.P., Lombardero M.J. (1999). Climate and the northern distribution limits of Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). Journal of Biogeography 26 , 1133-45.
[14] Faucet C., Rousselet J., Pineau P.; Miard F., Roques A. (2013). Are heat waves susceptible to mitigate the expansion of a species progressing with global warming? Ecology and Evolution 3 (9), 2947-57.
[15] Parmesan C, Ryrholm N, Stefanescu C et al (1999). Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399 , 579-83.
[16] Parmesan C (1996). Climate change and species’ range. Nature 382 , 765-766.
[17] Hickling R., Roy D.B., Hill J.K., Fox R., Thomas C.D. (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology 12 , 450- 455.
[18] Trân J.K., Ylioja T., Billings R.F., Régnière J., Ayres M.P. (2007). Impact of minimum winter temperatures on the population dynamics of Dendroctonus frontalis . Ecological Applications 17 , 882-899.
[19] Jepsen J.U., Hagen S.B., Ims R.A., Yoccoz N.G. (2008). Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. Journal of Animal Ecology 77 , 257-264.
[20] Chapman J.W., Reynolds D.R., Smith A.D., Riley J.R., Pedgley D.E., Woiwod I.P. (2008). Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Current Biology 18 , 514-518.
[21] Battisti A. Stastny M., Buffo E., Larsson S. (2006). A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Global Change Biology 12 , 66- 671.
[22] Vanhanen H., Veteli T.O., Pailvinen S., Kellomaki S., Niemala P. (2007). Climate change and range shifts in two insect defoliators: Gypsy moth and nun moth – A model study. Silva Fennica 41 , 621-38 ;
[23] Bale J.S., Masters G.J., Hodkinson I.D. et al. (2002). Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology 8 , 1-16.
[24] Eschen R., Roques A., Santini, A. (2015). Taxonomic dissimilarity in patterns of interception and establishment of alien arthropods, nematodes and pathogens affecting woody plants in Europe. Diversity and Distributions 21 (1), 36-45.
[25] Walther G R., Roques A., Hulme P.E., Sykes M.T., Pyšek P., et al. (2009). Alien species in a warmer world: risks and opportunities. Trends in Ecology and Evolution 24 , 686-692.
[26] Roques A. (2010). Alien forest insects in a warmer world and a globalized economy: Impacts of changes in trade, tourism and climate on forest biosecurity. New Zealand Journal of Forestry 40 , 77-94.
[27] Ott J., ed (2008). Monitoring climate change with dragonflies. Series Faunistica 81. Pensoft, Sofia. Cannon R.J.C. (1998). The implications of predicted climate change in the UK, with emphasis on non-indigenous species. Global Change Biology 4 , 785- 796.
[28] Eritja R., Escosa R., Lucientes J. et al. (2005). Worldwide invasion of vector mosquitoes: Present European distribution and challenges for Spain. Biological Invasions 7 , 87-97.
[29] Newman J.A. (2005). Climate change and the fate of cereal aphids in Southern Britain. Global Change Biology 11 , 940-944.
[30] R.J.C. Cannon (1998). The implications of predicted climate change in the UK, with emphasis on non-indigenous species. Global Change Biology 4 , 785-796.
[31] Auger-Rozenberg M.A., Barbaro L., Battisti A., Blache S.,. Charbonnier Y., et al. (2015). Ecological responses of parasitoids, predators and associated insect communities to the climate-driven expansion of pine processionary moth. pp. 311- 358 In Roques A. (Ed.) Processionary Moths and Climate Change: An Update. Springer/ Quae.
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie ( www.a3e.fr ), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: ROQUES Alain, AUGER-ROZENBERG Marie-Anne (February 23, 2024), 气候变化和全球化,昆虫入侵的驱动力, Encyclopedia of the Environment, Accessed August 24, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/zh/vivant-zh/climate-change-globalization-drivers-of-insect-invasions/ .
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.
Author(s)
Article outline
- 1. 欧洲非本地昆虫种群建立的爆炸式增长
- 2. 非本地物种在欧洲的传播速度越来越快
- 3. 全球变暖正在改变昆虫的分布范围
- 4. 年平均气温的升高,是一个与之有关的变量吗?
- 5. 北扩南缩?
- 6. 全球化与全球变暖协同作用
- 7. 仅仅全球变暖是造成昆虫扩散的原因吗?
- 8. 重要的信息(Messages to remember)
Learn more
The path of carbon in photosynthesis
04-03-2020
How is the carbon from carbon dioxide – CO2 – present in the atmosphere integrated…
Jean-François MOROT-GAUDRY , INRA Emeritus Research Director, the Jean-Pierre Bourgin Institute, INRA Versailles, Member of the Académie d’Agriculture.